|
14.09.2017 22:33:00
|
Pear Therapeutics Obtains FDA Clearance of the First Prescription Digital Therapeutic to Treat Disease
BOSTON and SAN FRANCISCO, Sept.14, 2017 /PRNewswire/ -- Pear Therapeutics, the leader in a new era of prescription digital therapeutics, today announced that the U.S. Food and Drug Administration (FDA) has granted Pear's De Novo request, allowing the company to market reSET® for the treatment of patients with substance use disorder (SUD) under a new class of treatment. This is the first time that the FDA has cleared a Prescription Digital Therapeutic with claims to improve clinical outcomes in a disease.
"This is a defining moment for digital therapeutics and for patients with substance use disorder," said Corey McCann, President and Chief Executive Officer of Pear Therapeutics. "As the first FDA-cleared Prescription Digital Therapeutic for disease treatment, reSET® has demonstrated improved abstinence and treatment retention in a randomized controlled clinical study. We believe that prescription digital therapeutics hold promise in improving patient outcomes across a wide range of central nervous system disorders including psychiatry, neurology and pain, and will become a vital part of tomorrow's treatment paradigm across all disease areas. Pear was impressed by the collaborative approach the FDA took in reviewing this innovative technology."
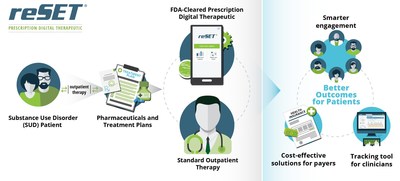
reSET® is a 12-week duration, FDA-cleared Prescription Digital Therapeutic to be used in conjunction with standard outpatient treatment for substance use disorder related to stimulants, cannabis, cocaine, and alcohol. reSET® is not intended to be used to treat opioid dependence. The product combines patient-facing interventions and assessments via a mobile device, with clinician-facing dashboards and data analytics on the back-end. reSET® has been proven to increase abstinence from a patient's substances of abuse during treatment and increase patient retention in the outpatient treatment program. reSET® provides access to self-reported substance use, triggers, cravings and outcomes to the patient's medical provider.
To support the FDA submission of reSET® (academic name: TES), a National Institute on Drug Abuse (NIDA) –sponsored clinical trial evaluated the therapeutic in 399 patients with SUD across 10 treatment centers in NIDA's Clinical Trial Network nation-wide over 12 weeks. Patients were randomized to either a standard treatment-as-usual, which consisted of standard face-to-face counseling, or to a reduced amount of face-to-face counseling plus the digital therapeutic. The clinical study demonstrated that the digital therapeutic more than doubled the rate of abstinence compared to standard, face-to-face counseling. In a sub-group analysis of non-abstinent patients at study start, a poor prognostic indicator, patients randomized to the digital therapeutic demonstrated an almost five-fold improvement in abstinence.
"In 2016, an estimated 20.1 million people aged 12 or older needed substance use disorder treatment according to the Substance Abuse and Mental Health Services Administration," said Edward V. Nunes, MD, Professor of Psychiatry at Columbia University Medical Center and independent Lead Investigator on the clinical study submitted to the FDA. "The clinical outcomes demonstrated in the reSET® pivotal study are remarkable. Clinically-validated digital therapeutics may become a cornerstone of future treatment."
"reSET® has the opportunity to radically enhance the treatment options for patients with substance use disorder. These digital therapeutics equip clinical professionals with the ability to provide more immediate and convenient care to their patients," said Advisory Board Chairman for Pear Therapeutics and Former Congressman, Patrick J. Kennedy. "I am proud to support Pear Therapeutics and their commitment to develop technologies for those living with mental health and substance use disorders."
About Substance Use Disorder (SUD)
In 2016, approximately 20.1 million people aged 12 or older had a substance use disorder (SUD) related to their use of alcohol or illicit drugs in the past year. Abuse of and addiction to alcohol, nicotine, and illicit and prescription drugs cost Americans more than $700 billion a year in increased health care costs, crime, and lost productivity, and contribute to the death of more than 90,000 Americans.
reSET® Indications for Use
reSET® is intended to provide cognitive behavioral therapy, as an adjunct to a contingency management system, for patients 18 years of age and older who are currently enrolled in outpatient treatment under the supervision of a clinician. reSET® is indicated as a 12-week prescription-only treatment for patients with substance use disorder (SUD), who are not currently on opioid replacement therapy, who do not abuse alcohol solely, or who do not abuse opioids as their primary substance of abuse. It is intended to:
- increase abstinence from a patient's substances of abuse during treatment, and
- increase retention in the outpatient treatment program.
Full prescribing information can be found at www.peartherapeutics.com.
About Prescription Digital Therapeutics
Prescription digital therapeutics are clinically validated, FDA-cleared software applications that demonstrate safety and efficacy in randomized clinical trials to improve patient outcomes. They are designed to enhance clinical outcomes, and where clinically relevant may be combined with current treatment regimens including approved drug or device therapies. Prescription digital therapeutics usually include patient-facing applications, clinical assessment and outcomes tracking, clinician monitoring dashboards and HIPAA-compliant data storage.
About Pear Therapeutics
Pear Therapeutics is the leader in FDA-cleared Prescription Digital Therapeutics. The company's approach is to integrate clinically-validated software applications with previously approved pharmaceuticals and treatment paradigms to provide better outcomes for patients, smarter engagement and tracking tools for clinicians, and cost-effective solutions for payers. Pear's lead product, reSET®, is an FDA-cleared 12-week interval prescription therapeutic for Substance Use Disorder (SUD) to be used as an adjunct to standard, outpatient treatment. Pear's product development pipeline includes reSET®-O™ for opioid use disorder (OUD) and additional prescription digital therapeutics in schizophrenia (Thrive™), combat posttraumatic stress disorder (reCALL™), general anxiety disorder (reVIVE™), pain, major depressive disorder, and insomnia, for which Pear intends to obtain FDA clearance. For more details, please see www.peartherapeutics.com.

View original content with multimedia:http://www.prnewswire.com/news-releases/pear-therapeutics-obtains-fda-clearance-of-the-first-prescription-digital-therapeutic-to-treat-disease-300520068.html
SOURCE Pear Therapeutics
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!