|
15.06.2022 20:00:00
|
Fecal Transplants Appear Crucial to Protecting Newborns After Receiving Antibiotics
Study from Cincinnati Children's details how antibiotics disrupt healthy gut microbiota, why this interferes with the lung's immune system, and how elevated risk of fatal pneumonias can be reduced
CINCINNATI, June 15, 2022 /PRNewswire/ -- Antibiotics are among the most powerful superheroes of medicine. From the introduction of penicillin in the 1930s to the dramatic rescues that occur today when doctors use strong antibiotics like vancomycin to combat "superbugs," many people know that bacteria-slaying antibiotics have saved uncounted lives from deadly infections.
Less well known: the price that some children appear to pay when the early use of strong antibiotics disrupts how their immune systems develop at a fundamental, potentially life-long level. When given "just in case," the cost of antibiotic use can be higher than many realize: in the forms of children being unable to fight off infection-triggered pneumonias later in childhood, becoming more likely to develop asthma and other conditions that can become life-long chronic health problems.
Cincinnati Children's neonatologist and immunology expert Hitesh Deshmukh, MD, PhD, has been studying this issue for years. Now his research team has completed another important study that documents why doctors should be concerned about the near-casual overuse of antibiotics. Details were published online June 15, 2022, in Science Translational Medicine.
"It is not possible to completely discontinue the use of antibiotics in newborns. In certain cases, antibiotics are the only way to prevent death from infections like Group B Streptococcus," says Deshmukh. "However, this paper shows why antibiotic use must be kept to the absolute minimum and why it's important to compensate for that usage as soon as possible."
Balancing the good vs. the bad in antibiotic treatmentAbout 1 in 4 pregnant women carry Group B streptococcus (GBS) in their bodies without showing any obvious symptoms. While these bacteria rarely causes severe problems for adults, infections can cause sepsis, pneumonia and meningitis in newborns, any of which can be fatal without skilled hospital care.
In the US, approximately 7,600 GBS infections in newborns occurred per year before widespread use of preventative antibiotics, according to the federal Centers for Disease Control and Prevention. Since 1993, the rate of infections occurring in the first week after birth has decreased about 87%, from 1.7 cases per 1,000 live births to 0.22 cases per 1,000 live births in 2016.
However, more than 2.4 million babies are born via vaginal delivery in the US each year. In the 1990s, as many as half of those women were receiving just-in-case antibiotic treatment. Since 2002, when screening tests for GBS became standard recommended practice, the use of preventative antibiotics has tightened.
Still, with as many as 500,000 newborns a year in the US being exposed to immune-disrupting antibiotics, Deshmukh says more attention must be given to the large-but-unknown number of children experiencing the side effects—including elevated risks of developing severe pneumonia later in infancy and early childhood, as well as life-long health problems like asthma.
Deep Relationship Between Gut Bacteria and Lung DevelopmentA growing body of research, including work led by experts at Cincinnati Children's, has gradually uncovered how extensively the mix of bacteria and fungi that normally live in our intestines influences our health. In 2017, Deshmukh published findings, based on mouse models, that demonstrated how antibiotics go far beyond killing "bad" bacteria to disrupt the still-developing immune system of newborns by also killing "good" bacteria.
In this study, animals that were exposed to antibiotics as newborns and later exposed to bacteria known to trigger severe pneumonia were compared to animals that were not exposed to early antibiotic treatment. All of the antibiotic-disrupted animals suffered severe symptoms within 60 hours. All of those with undisrupted immune systems avoided severe symptoms.
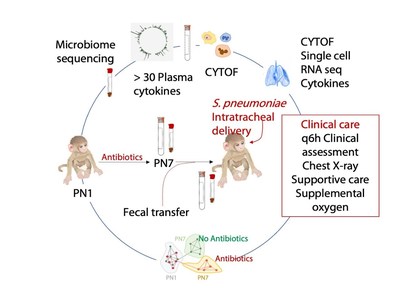
This research dove especially deep into the molecular and genetic differences that occur between newborns that receive early antibiotic therapy and those who do not. The 17-member research team, led by Deshmukh and first author Joseph Stevens, an immunobiology graduate student, used single-cell RNA sequencing, flow cytometry, a growing atlas of genetic data called LungMAP, and other resources to analyze several types of immune cells in the lungs, plus the complex mix of bacteria in the gut. The study took two years to complete.
The team identified a host of disruptions caused by antibiotic exposure in both the amount and structure of immune cells called neutrophils found in the blood and in lung tissue. They also documented damaged versions of other immune cell types including T-cells, alveolar macrophages, and interstitial macrophages. All of these changes combined to produce a hyperinflammatory response in the lungs to infection among the antibiotic-exposed newborns.
Some of these dysfunctional cells already have been found in human newborns that suffered severe pneumonia after various types of infections that many other children recover from with ease.
These details will be important to scientists looking for ways to repair the damage that antibiotic use can cause in newborns. For example, the co-authors say they have found potential biomarkers, or molecular signatures, that could rapidly reveal which children are at highest risk of developing severe pneumonia. This could allow clinicians to intervene more aggressively for those children when infections strike.
Eventually, this line of work may lead to more precise use of antibiotics, re-designed antibiotics that do less harm to "good" bacteria, and supportive treatments to restore healthy gut microbiota when powerful antibiotic treatment is required.
"The body moves quickly after birth to fully establish the lung's immune protection system," Deshmukh says. "Failure to restore healthy gut microbiota before that development window closes can result in infants growing up with lungs that are permanently less able to respond to infections later in life."
More science behind fecal transplantationThe good news from the latest findings is that the damage antibiotics done to commensal microbiota can be restored by transferring a supply of healthy bacteria to the intestines of a child that lacks them – a process called a fecal transplant.
In this study, fecal transplants fully restored commensal bacteria to normal pre-exposure levels in some animals that had early antibiotic damage, but only partially in others. Those with restored and partially restored gut microbiota went on to develop stronger immune systems in their lungs and were better able to resist the pneumonia-causing infection "challenge."
Several scenarios may explain this partial restoration, the co-authors wrote. But more research is needed to confirm which possibilities are the most accurate explanation.
What's the takeaway for families?It may take years to make fecal transplantation a common practice in newborn care, if indeed future human studies prove that such transplants are needed. In the meantime, what should families do to minimize potential risks to their child's longer-term health that might be linked to a dose of antibiotics at birth?
First, Deshmukh says parents should ask questions until they are satisfied they understand why the doctor is recommending the antibiotic. These medications remain highly necessary for treating serious, potentially fatal infections.
Currently, fecal transplantation is being studied in a number of clinical trials nationally–but primarily as a supportive therapy for people receiving gut-disrupting treatments, such as stem cell transplants for cancer. So far, one small study published in 2020 by scientists in Denmark has found that fecal transplantation from mother to child after a C-section rapidly caused the newborn's gut microbiota to match their mothers'.
Deshmukh says Cincinnati Children's is not performing fecal transplants for otherwise healthy newborns, even in clinical trials, and he is unaware of any other hospitals doing so.
It may also be possible to re-balance a child's commensal bacteria in healthy ways through other methods, such as adding back molecules that are made by normal, healthy microbiota. However, more work is needed to identify the specific players needed for these more targeted strategies. Currently, the most useful longer-term way to reduce the chances of contracting a dangerous infection that could lead to pneumonia is for children and adults to receive all the vaccines they qualify for.
Next stepsFurther study remains required to fully translate the animal-based findings into changes in practice among neonatologists, new tests, or improved antibiotics. Beyond complete fecal transplants that transfer a small amount of every bug in a healthy gut, researchers also hope to determine which types of bacteria are most important for transfers.
"Our study lacked the power to establish a clear association between specific bacterial taxa and clinical response to pneumonia," the co-authors state.
More study also is needed to determine what impact, if any, does breastmilk have on immune protection amid the chaos triggered by antibiotic exposure. Breastmilk is known to confer considerable immune protection to newborns thanks to super antibodies produced by mothers, but it is not clear how strongly that benefit outweighs the disruption triggered by early antibiotics. All the animals were formula fed in this study.
About this studyFunding for this research (DOI number: 10.1126/scitranslmed.abl3981) includes several grants from the National Institutes of Health supporting co-authors and core laboratory resources (HD084686, HL155611, ES029234, HL142708, ES029234, AG053498, HD028827, HL149366, AI152100, CA226802, HL148865, HD89939, EB029863, GM128452, AI138553, HL142485, AI157626, AI150554, P51 OD11107, OD27094, U01HL134745 and U01HL122642)
The authors declare they have no competing interests.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/fecal-transplants-appear-crucial-to-protecting-newborns-after-receiving-antibiotics-301568873.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/fecal-transplants-appear-crucial-to-protecting-newborns-after-receiving-antibiotics-301568873.html
SOURCE Cincinnati Children's Hospital Medical Center
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!